
GMP Document Management Documentation Hierarchy API FIRST
A QMS is a set of documents and processes that help an organization bring products to market that are safe and effective, meet regulatory requirements, and consistently meet customer expectations. What is the definition of quality? Given that we're defining the quality system, it may be useful to understand the meaning of quality in this context.
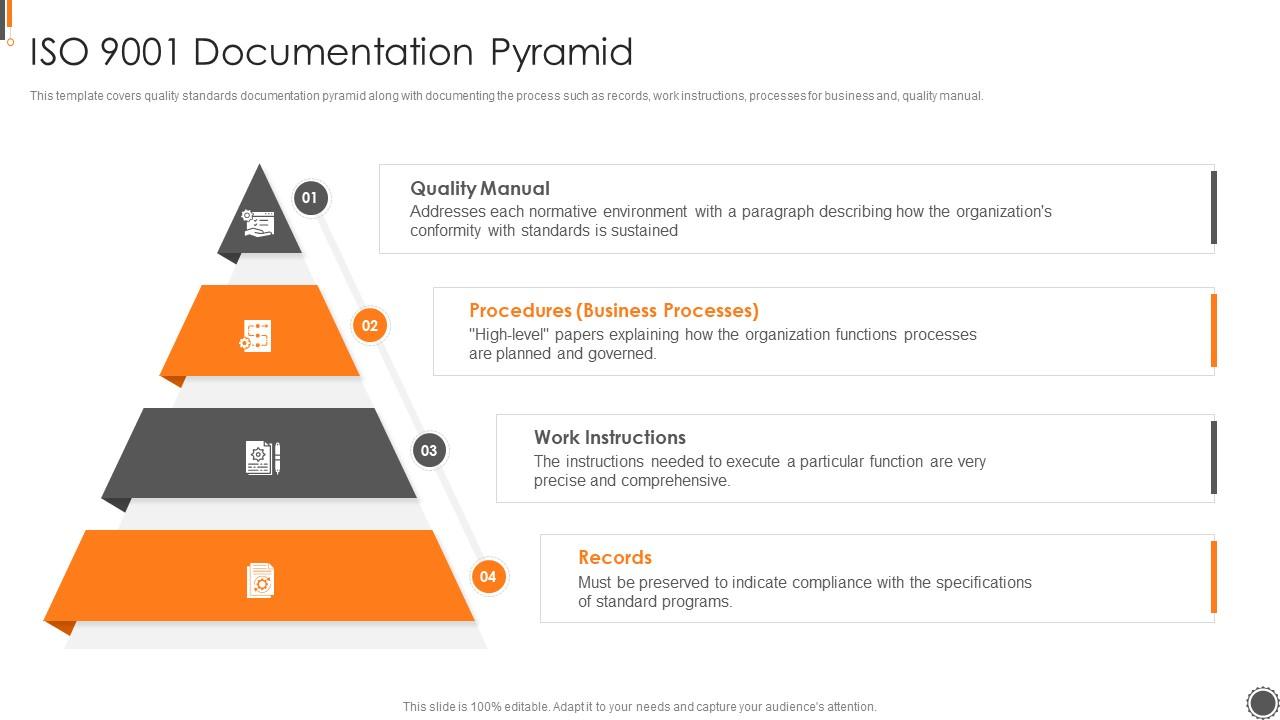
ISO 9001 Documentation Pyramid ISO 9001 Certification Process Ppt Sample Presentation Graphics
Previous Article. A Quality Management System (QMS) is a formalized system documenting processes, procedures, and responsibilities for ensuring consistent delivery of high-quality products and services to the consumer. A QMS provides a framework for employees to plan their work, monitor progress, identify problems, and take corrective action.
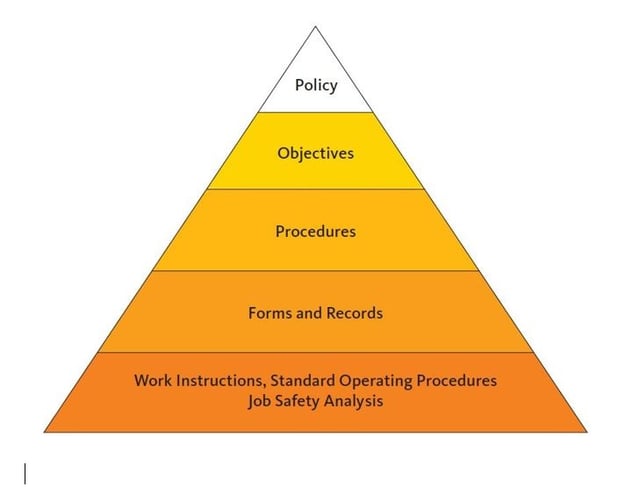
How to Implement a QMS and Achieve ISO 9001 Certification
A quality management system (QMS) is defined as a formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives. A QMS helps coordinate and direct an organization's activities to meet customer and regulatory requirements and improve its effectiveness and efficiency on a continuous.

Quality System DocumentationPresentationEZE
The quality manual is the document that identifies and describes the QMS. The adoption of a QMS should be a strategic decision of an organization. The design and implementation of an organization's QMS (documentation) is influenced by 1. its organizational environment, changes in that environment, and the risks associated with that environment,

Quality Priority Pyramid Brewers Association
The Pyramid model is described as a tool to manage the quality systems. Finally, some experiences in other countries are given to prove the validity of the system. Keywords: Pyramid model, quality system, quality assurance, Good Manufacturing Practice (GMP), ISO 9001, GMP for blood banks, quality policy and strategy Go to: Introduction
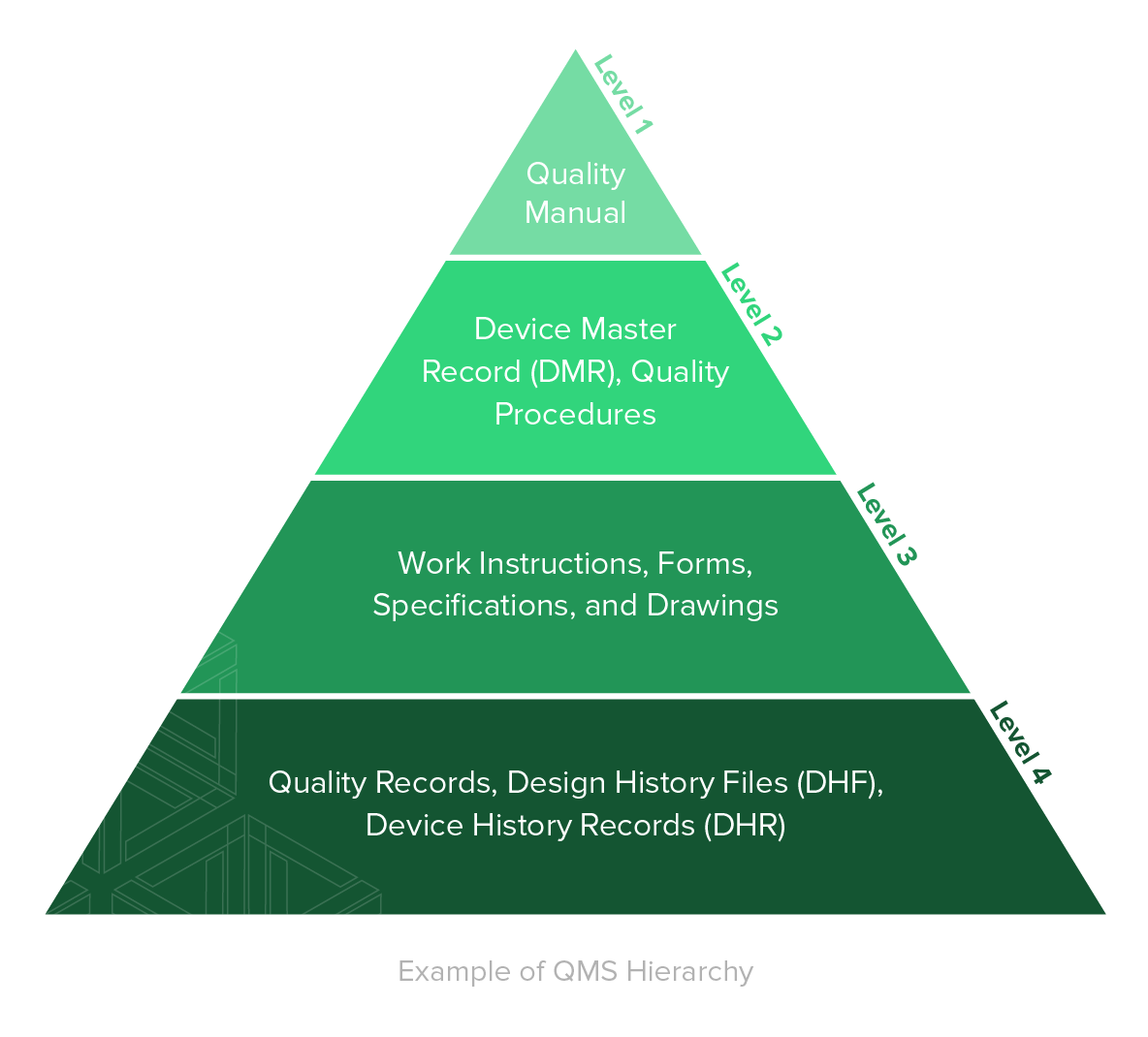
Ultimate Guide to ISO 13485 Quality Management System (QMS) for Medical Devices
Pyramid of the quality system documentatio n This is achieved by the following measures and activities: Manage documents on the basis of written procedures Ensuring that the valid document.
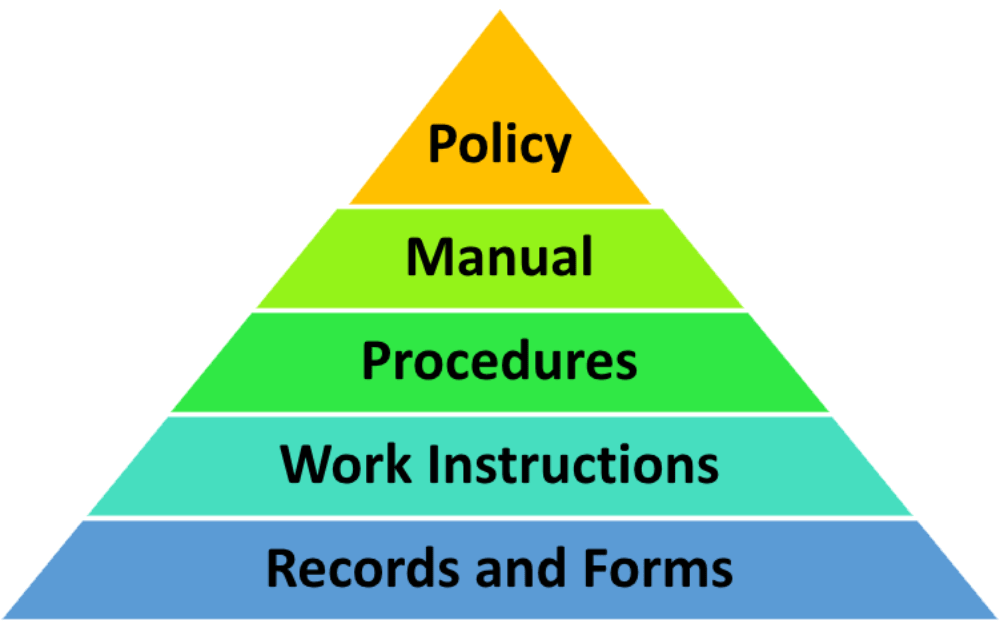
ISO 9001 QMS documentation How to structure it
Abstract. Three quality systems that can be used in blood establishments are briefly explained. The Pyramid model is described as a tool to manage the quality systems. Finally, some experiences in.
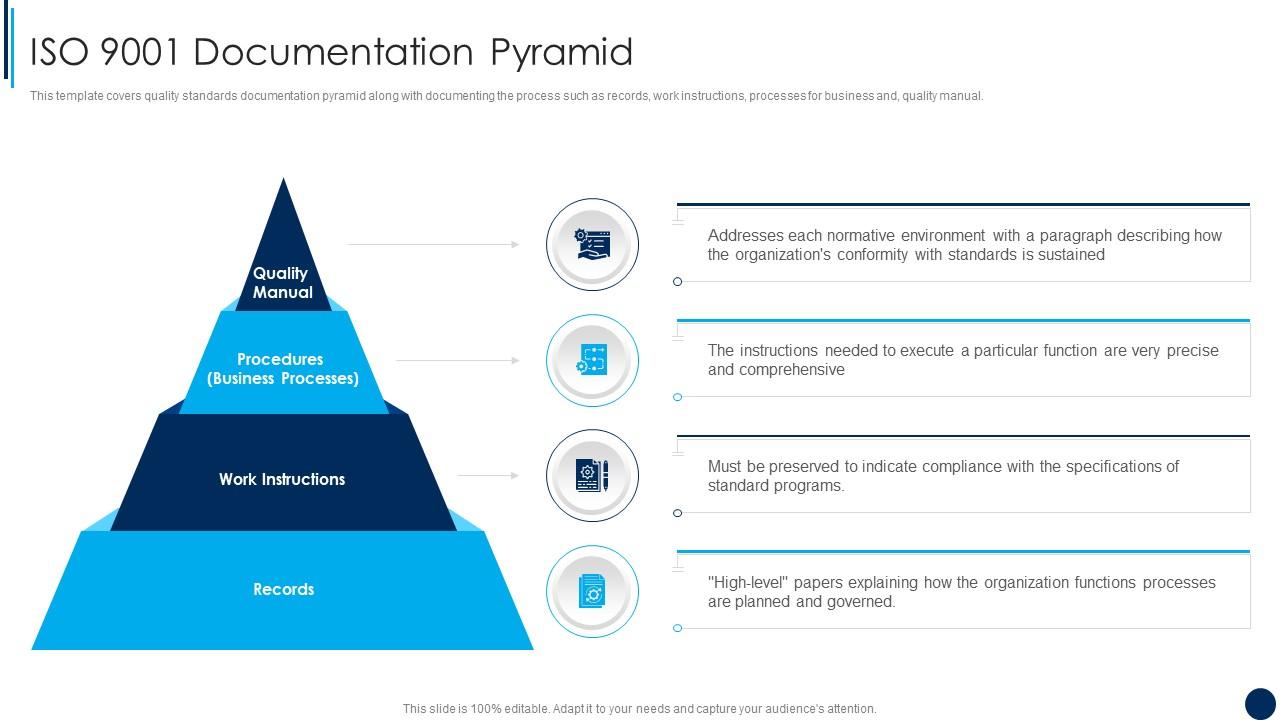
ISO 9001 Documentation Pyramid ISO 9001 Quality Management Ppt Demonstration Presentation
Article Full-text available Dec 2010 Milan Eri Vladimir Nedic Miladin Z Stefanovic Marko Djuki One of the basis of a series of standards JUS ISO 9000 is quality system documentation. An.
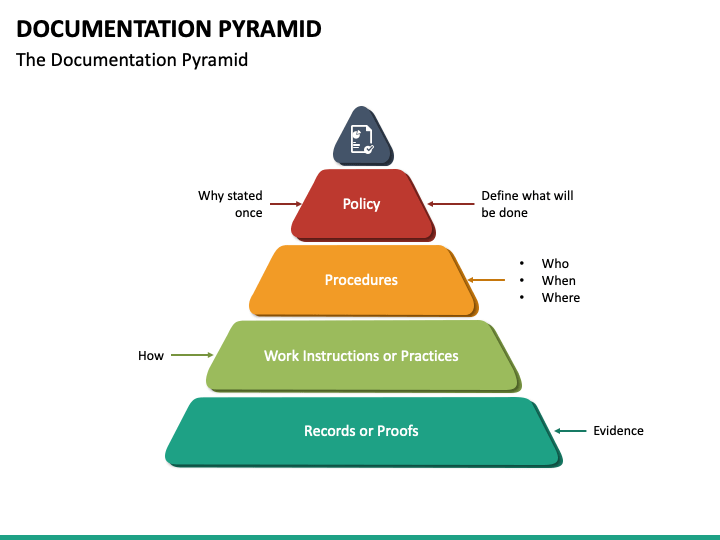
Quality Management System Pyramid
The four-tier operational pyramid does emphasize the impact of the quality manual (manual) on the entire documentation structure, although—as noted—the pyramid is only meant to be a guideline because it does not replace the actual linkage that must be present from document to supplemental document. 4.2.5 Navigation Is Key
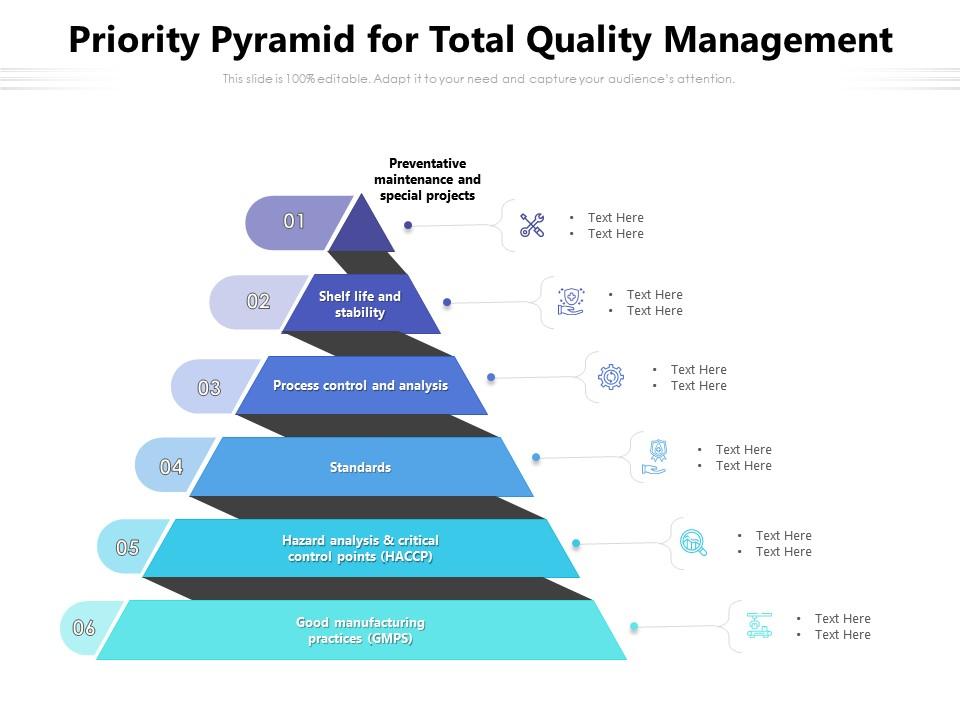
Priority Pyramid For Total Quality Management Presentation Graphics Presentation PowerPoint
A quality management system (QMS) is a set of policies, procedures, and processes that define how an organization ensures the quality of its products and services. A QMS document.
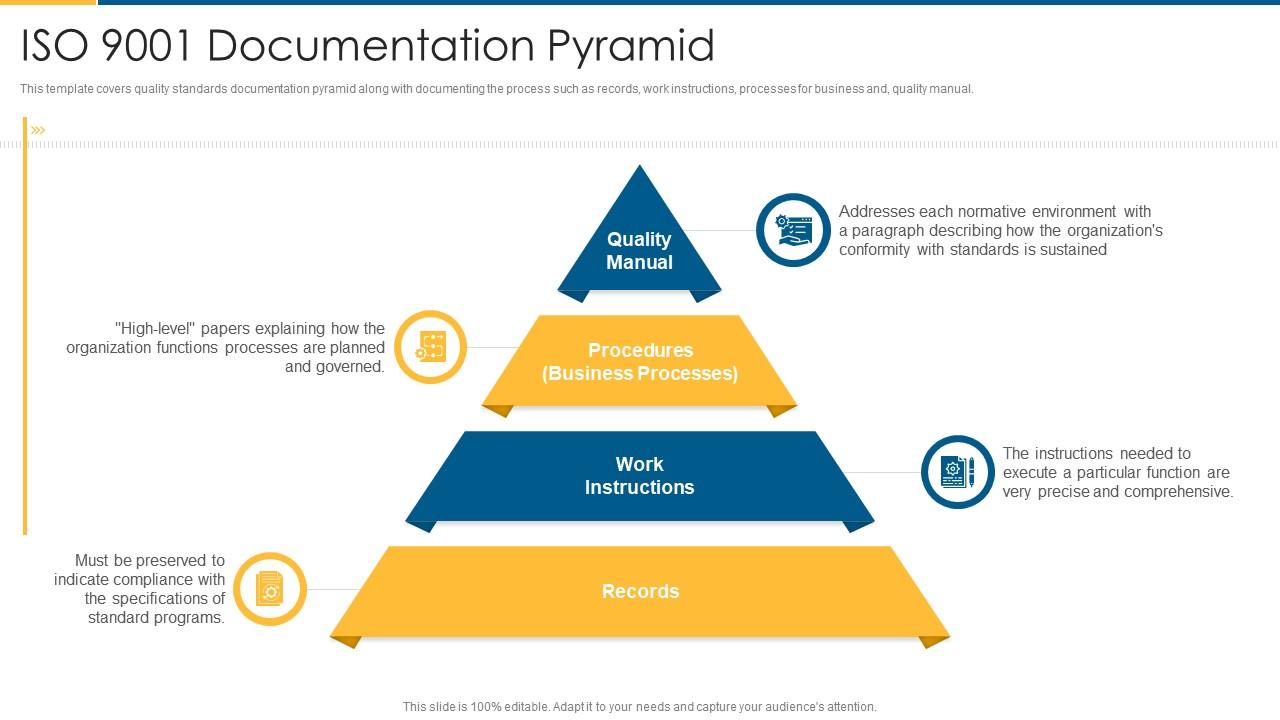
Iso 9001 Iso 9001 Documentation Pyramid Presentation Graphics Presentation PowerPoint
A Quality Manual Documented Procedures Work Instructions With ISO 9001:2015, it is now left up to the organization to determine which documents it believes it needs (considering factors such as "interested parties," "organizational knowledge," etc.).
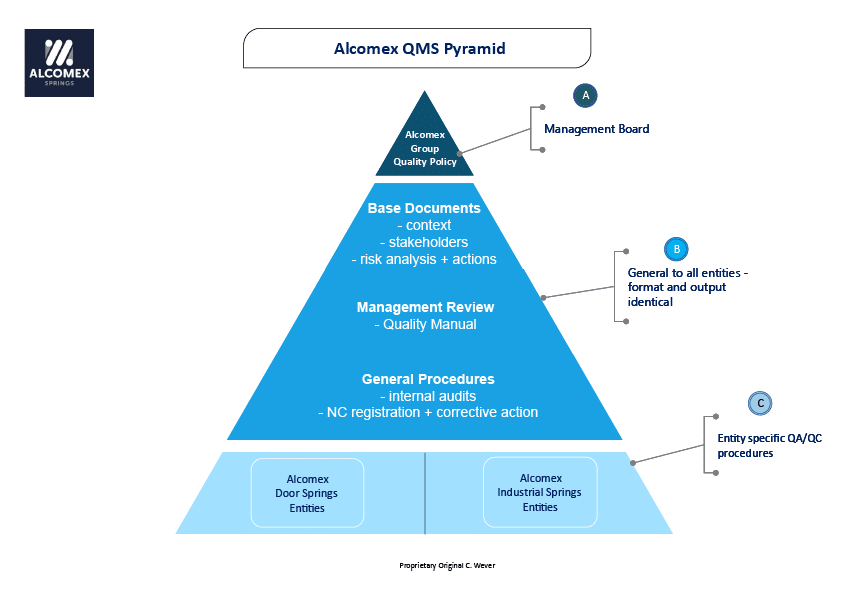
The Quality Management System pyramid news
Here's how to structure your IAF 16949 Quality Management System documentation: 1) Quality manual. The quality manual needs to be tailored to your company - its structure and content should be based on your company size, operational complexity, and employee competence. Smaller companies will be able to fit their whole QMS into one manual.

Quality Management System Documentation Quality Gurus
Quality System Documentation is a pyramid in shape. The Quality Manual, the top level document, links the quality system procedures, work instructions and forms via applicable document references. Be aware of when a company is audited, the auditors will use your Quality Manual as the audit document. The drawing shown below provides the general.
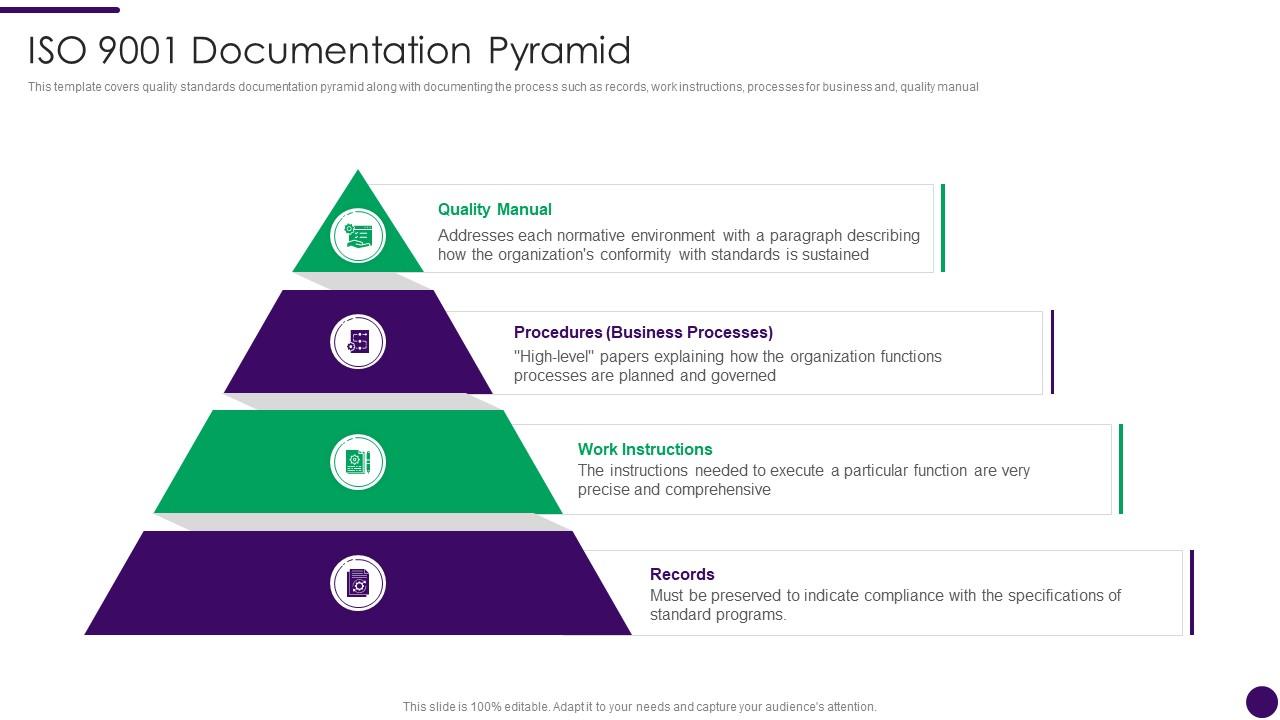
ISO 9001 Documentation Pyramid How To Achieve ISO 9001 Certification Ppt Ideas Presentation
The QMS documentation hierarchy is presented in the diagram below: ISO 9001 requires different types of documents however, they do not need to be written as separate documents. The standard is flexible, so that the organization can decide on the size of the documents and the level of details documented.
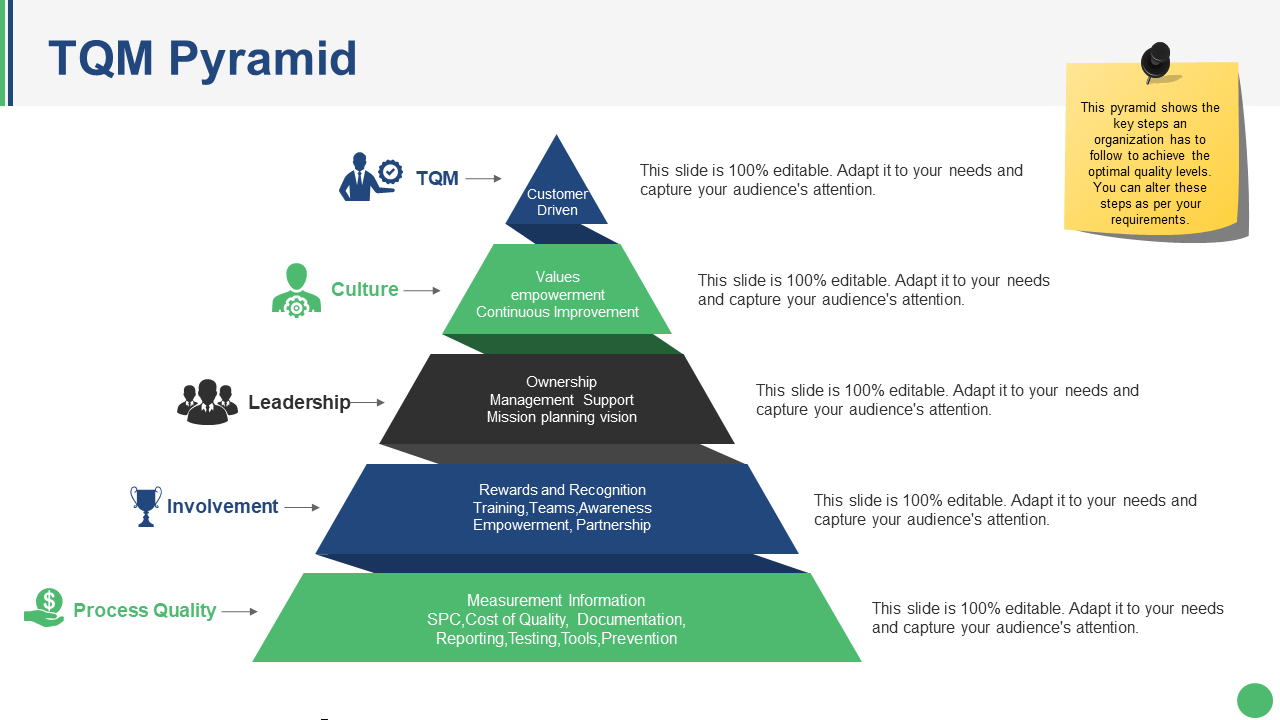
21 High Quality Templates For Your Quality Management
implement, and maintain a quality management system and continually improve its effectiveness in accordance with the requirements of this International Standard" Clause 4.2.1 General explains that the quality management system documentation shall include: a) documented statements of a quality policy and quality objectives; b) a quality manual
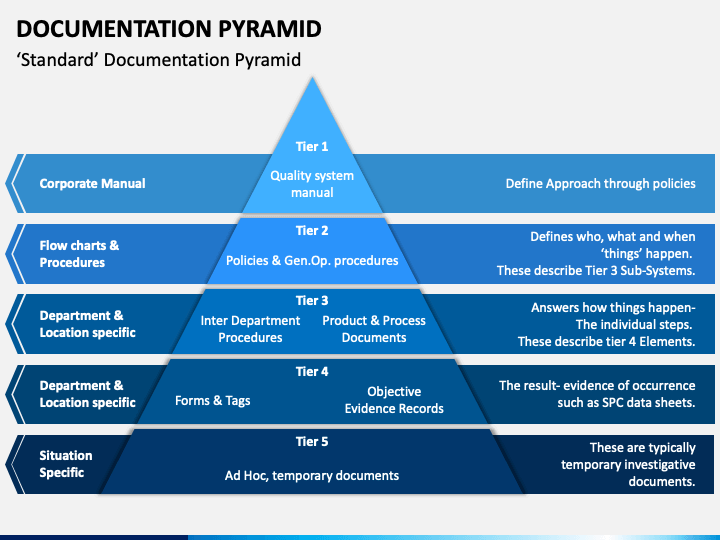
Quality Management System Pyramid
QMS documentation hierarchy A typical QMS will contain a variety of documents, some of which include: Quality Policy Quality Manual Procedures and Work Instructions Quality Plans and Records The hierarchy of this QMS documentation is depicted in the diagram below: